- 11.88 MB
- 2022-08-18 发布
- 1、本文档由用户上传,淘文库整理发布,可阅读全部内容。
- 2、本文档内容版权归属内容提供方,所产生的收益全部归内容提供方所有。如果您对本文有版权争议,请立即联系网站客服。
- 3、本文档由用户上传,本站不保证质量和数量令人满意,可能有诸多瑕疵,付费之前,请仔细阅读内容确认后进行付费下载。
- 网站客服QQ:403074932
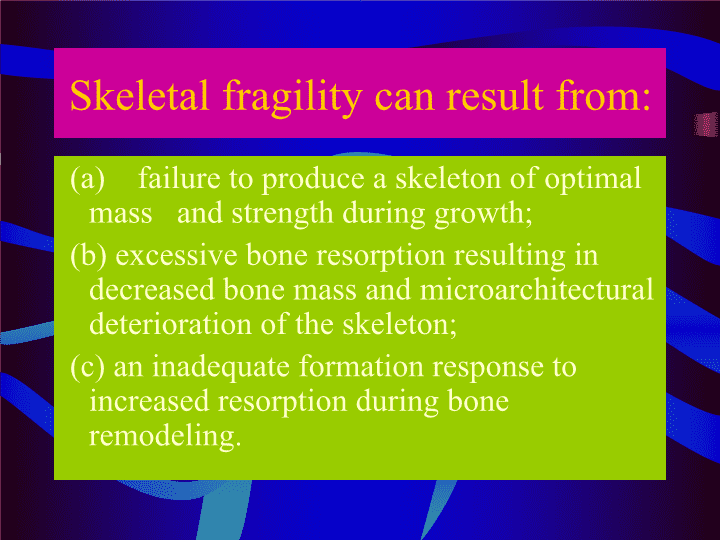
周后德2005.4.20长沙InstituteofMetabolism&Endocrinology,CentralSouthUniversity代谢性骨病细胞、分子生物学基础及研究方法与策略\n代谢性骨病代谢性骨病是一类伴有钙、磷、维生素D代谢失调以及甲状旁腺功能、细胞因子与激素分泌异常的全身性骨骼病[代谢性骨病的分类]1.骨质疏松2.佝偻-软骨病3.原发性甲状旁腺机能亢进症4.原发性甲状旁腺机能减退症5.中毒性骨病(维生素D、氟、镉、铅、磷等中毒)6.其他如粘多糖代谢异常,Marfan症,Paget症,SEDT-PA。osteoporosis:Representsacontinuum,inwhichmultiplepathogeneticmechanismsconvergetocauselossofbonemassandmicroarchitecturaldeteriorationofskeletalstructure.Thesefactors,coupledwithanincreasedriskoffalls,contributetoahighincidenceoffragilityfracturesinosteoporoticpatients.\nSkeletalfragilitycanresultfrom:(a)failuretoproduceaskeletonofoptimalmassandstrengthduringgrowth;(b)excessiveboneresorptionresultingindecreasedbonemassandmicroarchitecturaldeteriorationoftheskeleton;(c)aninadequateformationresponsetoincreasedresorptionduringboneremodeling.\nTheboneremodelingunitsorbonemulticellularunits(BMUs)\n骨组织的细胞成份成骨谱系:间充质干细胞,骨源性始组细胞(osteoprogenitorcells)、成骨细胞(osteoblast)、骨衬细胞(bone-liningcell)、骨细胞(osteocyte)破骨谱系:多潜能造血干细胞、破骨细胞(osteoclast)成软骨细胞、软骨细胞、破软骨细胞InstituteofMetabolism&Endocrinology,CentralSouthUniversity\n非细胞成分胶原非胶原蛋白骨矿物质血管神经InstituteofMetabolism&Endocrinology,CentralSouthUniversity\n组织细胞学基础InstituteofMetabolism&Endocrinology,CentralSouthUniversityOsteoblastdevelopmentanddifferentiation\nTranscriptionfactorsCbfa1/Runx2++++++?SurfacemarkersSTRO-1+++/----SB-10/ALCAM00----HOP-26/CD63++----EnzymesAlkalinephosphatase---++++++-Phex----++++Collagenandnon-collagenousproteinsCollagentypeI?-+++++++-Osteocalcin-----+++-Bonesialoprotein--++-+++++++++Osteopontin+?-/+-/+-/+-+++++++\nEffectsofWntsignalingonosseouscells.ThecanonicalWntsignalingpathwaypromotestheproliferation,expansionandsurvivalofpre-andimmatureosteoblasts.Dkks,Sfrps,andWif-1antagonizeWntsignalinginosteoblaststofacilitatedeathofimmaturecells,buttheymayalsodownregulatethepathwayinmaturecellstoinduceterminaldifferentiation.\nWntsignalinginosteoblastsandbonediseases\nOsteoclastdevelopmentanddifferentiationNormaldifferentiationrequires:tyrosinekinase-andtumornecrosis-familyreceptors,normallyfmsandRANK.Ligandsforthesereceptorsplusunidentifedserumorcell-presentedfactor(s)areneededforinvitrodifferentiation,possiblysignallingviaanimmune-liketyrosinekinaseacceptormolecule.Osteoclastdevelopmentandactivity:increasedbycytokinessignallingthroughGP130,suchasIL-6,byTGF-b,andbyIL-1.Othertyrosinekinasereceptorsincludingkitandmetcanaugmentfmssignalling,andTNFsotherthanRANKL,includingTNFaandTRAIL,modifyRANKsignalling.ThesituationisfurthercomplicatedbyG-proteincoupledreceptorsincludingthecalcitoninreceptor,byintegrinorcalcium-mediatedsignals,andbyestrogenreceptors,whichoperateinbonelargelyviaNOdownstreamsignals.Differentiation,activity,andsurvivalsignalsmergeinintracellularsecondmessengers:includecytoplasmickinasesofseveralfamilies;differentiationpathwaysoftenterminateinErk/JunkinasesorNF-jB.KeyregulatoryintermediatesincludeTRAF6,src,Smad3,phosphatidylinositol-3-kinase,Jak/Stat,andthecGMP-dependentproteinkinaseI.Therearesubstantialuncertaintiesregardinghowintracellularagentsconnecttoprimarysignals.\nInstituteofMetabolism&Endocrinology,CentralSouthUniversityOsteoclastdevelopmentanddifferentiation\nOsteoclastformationandactivity.ShowncentrallyisaschemeofosteoclastdifferentiationinresponsetoM-CSFandRANKL.Abovetheproposeddifferentiationpathwayareexamplesofdirectinhibitors,whilstbelowareindirectinhibitorssignallingthroughintermediatecelltypes.InstituteofMetabolism&Endocrinology,CentralSouthUniversity\n破骨细胞分化后的主要调节通路\nCell-to-cellinteractionsinboneBoneremodelingisadynamicprocess:requirescoordinatedcellularactivitiesamongosteoblasts,osteocytes,andosteoclastsAdherensjunctions:cadherinsinosteogenesisCommunicatingjunctions:GapjunctionsConnexin43,45,46,theporeisdifferentDevelopmentandmaintenanceofadifferentiatedosteoblastphenotype.activationoftheERKsignalingcascadepropagationofcalciumwaveInstituteofMetabolism&Endocrinology,CentralSouthUniversity\nThecadherinadhesioncomplex.InstituteofMetabolism&Endocrinology,CentralSouthUniversity\nAsingleconnexinmonomeriscomposedof4transmembranespanningdomains(TM1–4),twoextracellularloops(EC1,2),andacytoplasmicloop(CL).Aconnexon,orhemichannel,iscomposedofahexamericarrayofconnexins.Whendockedwithaconnexononanappositionalcellmembrane,agapjunctionalchannelisformedwhichpermitsthetransferofsmallmoleculessuchasnucleotides,metabolites,andsecondmessengersamongcoupledcells.\nCell–cellinteractionsplayanimportantroleinin.uencingandcoordinatingcellfunction.Bothadherensandcommunicativejunctionscanregulatetheabundanceormovementofsignalingmoleculesortranscriptionallyactivefactors,whosecanonicalpathwaysconvergeuponthenucleustomodulategeneexpressionandultimatelycellularfunction.\n各种细胞间的通讯与偶联InstituteofMetabolism&Endocrinology,CentralSouthUniversityChemicalsynapses,gapjunctions,Plasmodesmata,Tunnelingnanotubes\nModelofdeathligand/deathreceptorandmitochondria-mediatedclassicalapoptosissignaling,includingBcl-2members.InstituteofMetabolism&Endocrinology,CentralSouthUniversityCellApoptosis\nOsteoblast/osteocyteapoptosisapoptoticosteoblastswithtypicalapoptoticfeaturesofcondensedchromatinand/ornuclearfragmentationareseenrarelyinvivo(TUNEL+)theymustbejuxtaposedtoosteoidandtootherosteoblastsbecauseboneformationiscarriedoutbyteamsofosteoblasts,butnotinosteocytestheyshouldhaveacuboidalmorphologytodistinguishthemfromnearbymarrowcellsandliningcells;specifctechniques,suchasTUNELorISELstainingInstituteofMetabolism&Endocrinology,CentralSouthUniversity\n\nOsteoclastApoptosisInducedby:Bisphosphonatesandestrogenetal.Phenotype:cellshrinking,chromatincondensation,nuclearfragmentation,andstrongTRAPstainingInhibitby:Srcandintegrinsin,PU.1,c-Fos,NF-kBp50/p52,andNFATc1InstituteofMetabolism&Endocrinology,CentralSouthUniversity\nSummaryofstimulatoryandinhibitoryfactorsinvolvedinosteoclastdevelopmentandapoptosis.\nM-CSF,RANKL,TNF,andIL-1inducedsignalsleadingtoactivationofSrc,Akt,Erks,andmTOR/S6Kpathwaysinosteoclaststoblockapoptosis.InstituteofMetabolism&Endocrinology,CentralSouthUniversity\n成骨与破骨之间的藕联OPG/RANK/RANKLTraidIRSfamilyOthers(membraneadapterDNAX-activatingprotein12/Fcreceptorcommonγchain)\nRANKLisadendriticcellsurvivalfactor,butalsostronglyinducesosteoclastogenesisandboneresorptionviabindingtoRANK.OPGinhibitsosteolysisandblocksRANKL/RANKinteraction.AlthoughtheOPG/RANK/RANKLtriadisthemainmodulatorofboneapposition/resorptioncoupling,othercontrolsofosteoclasticdifferentiationalsoexist,suchasTNF-aandIL-1,whichareabletomodulatethebiologicalactivitiesofthetriad.\nTheinitialstepinRANKsignalingisthebindingoftheTRAF6adaptatorproteinwithinthecytoplasmicdomainofRANK.TRAF6thenactsasapivotaladaptorleadingtospecificgeneexpressionregulatingosteoclastdifferentiationandactivation.ThedownstreamtargetsofTRAF6includetranscriptionfactorssuchasNF-kB,aswellasmoleculesinvolvedinsignaltransduction.\n\nCentralcontrolofboneremodelingInstituteofMetabolism&Endocrinology,CentralSouthUniversity\n骨组织细胞功能研究方法InstituteofMetabolism&Endocrinology,CentralSouthUniversity\n骨组织细胞生物学行为分析InstituteofMetabolism&Endocrinology,CentralSouthUniversityMTTGrowthcurveCellcycle形成矿化结节的能力\nInstituteofMetabolism&Endocrinology,CentralSouthUniversity\n骨组织细胞的联合三维培养装置InstituteofMetabolism&Endocrinology,CentralSouthUniversity破骨细胞培养在骨片上形成骨吸收陷窝\nCombinedfluorescentandbright-fieldconfocalimagesillustratingpositivestainingforosteocytehypoxiaandactin.Innormalintactbone(A),minimalosteocytehypoxiawasdetected(1.1 ± 0.5%).After24 hofdisuse(B),osteocytehypoxiawassignificantlyelevated(8.4 ± 1.8%).Ahighlevelofpositiveactinstainingwasevident(C;72 ± 9%).InstituteofMetabolism&Endocrinology,CentralSouthUniversity\nInstituteofMetabolism&Endocrinology,CentralSouthUniversity基质干细胞的多向诱导性\n骨质疏松的其他分子机制osteoporosisislikelytobecausedbycomplexinteractionsamonglocalandsystemicregulatorsofbonecellfunction.Theheterogeneityofosteoporosismaybeduenotonlytodifferencesintheproductionofsystemicandlocalregulators,butalsotochangesinreceptors,signaltransductionmechanisms,nucleartranscriptionfactors,andenzymesthatproduceorinactivatelocalregulators.Withinthelastdecade,theidentificationofmanyoftheregulatorymechanismsthathavebeenlinkedtoosteoporosishasbeentheresultofgeneticstudies.\n骨质疏松的其他分子机制CentralroleofestrogenCalcium,vitaminD,andparathyroidhormoneReceptoractivatorofNF-κB,itsligand,andosteoprotegerinGenesdeterminingosteoblastdifferentiationandfunctionCytokines,prostaglandins,NO,andleukotrienesCollagenabnormalitiesLeptinandneuralpathways\n分子生物学研究基础及方法InstituteofMetabolism&Endocrinology,CentralSouthUniversity报告基因研究方法荧光示踪\n基因功能研究的其他手段转基因与基因剔除体外转录与翻译交互作用蛋白人工突变调控区研究InstituteofMetabolism&Endocrinology,CentralSouthUniversity\nInstituteofMetabolism&Endocrinology,CentralSouthUniversityMMP-1OPGBeta-ActinActinNorthernblotanalysisthechangeofMMPmRNAexpressioninosteoblasttreatedbyestrogenCVitcE2用Western分析雌激素与Vitc对成骨细胞OPG表达的影响Phospho-ERK1/2Total-ERK1/2Control16122448961,25D3(h)VitD3处理不同时间对细胞周期相关基因磷酸化的影响\nInstituteofMetabolism&Endocrinology,CentralSouthUniversity转录水平高通量分析方法用含8600个基因的芯片分析SEDT-PA患者成骨细胞基因表达谱的变化用含890个基因的细胞因子表达Array分析雌激素处理前后成骨细胞细胞因子表达谱的变化\nInstituteofMetabolism&Endocrinology,CentralSouthUniversity\nInstituteofMetabolism&Endocrinology,CentralSouthUniversityTheeffectofcbfa-1inthethedevelopmentofbones;alcianblue(forcartilage);alizarinred(forbone).A)Wild-typelittermate.(B)Homozygousmutantshowingcartilage,butanabsenceofossificationthroughouttheentirebody.ThetranslocationofP53inducedbyTNFisrepairedbyestrogendetectedbyIF\nImplicationforthediagnosisandtreatmentofosteoporosisPharmacotherapyforosteoporosishasbeenfocusedmainlyoninterventionsthatcouldreversethesecondpathogeneticmechanism,excessiveboneresorptionestrogenreplacementincreasedtheriskofbreastcancercalcitoninisaselectiveinhibitorofosteoclasticactivityBisphosphonatesbindtothebonesurfaceandthenaretakenupbyosteoclasts,leadingtotheirinactivationandprogrammedcelldeathlowdosesofPTHcouldincreasebonemassStrontiumranelatehasrecentlybeenapprovedfortreatmentofosteoporosisinseveralcountriesoutsidetheUSNewantiresorptiveapproachesarebeinginvestigated,includingselectiveinhibitorsofosteoclastichydrogeniontransportandcathepsinKaswellasantagoniststotheαvβ3integrinsthatarenecessaryforosteoclastadherenceandmotility.Newanabolicapproachesarelikelytoemergefromfurtherstudyofthetranscriptionalregulationofosteoblasts.\nInstituteofMetabolism&Endocrinology,CentralSouthUniversityGeneTherapyforOsteoinduction\nInstituteofMetabolism&Endocrinology,CentralSouthUniversity\nTHANKS!InstituteofMetabolism&Endocrinology,CentralSouthUniversity