- 445.83 KB
- 2022-04-26 发布
- 1、本文档由用户上传,淘文库整理发布,可阅读全部内容。
- 2、本文档内容版权归属内容提供方,所产生的收益全部归内容提供方所有。如果您对本文有版权争议,请立即联系网站客服。
- 3、本文档由用户上传,本站不保证质量和数量令人满意,可能有诸多瑕疵,付费之前,请仔细阅读内容确认后进行付费下载。
- 网站客服QQ:403074932
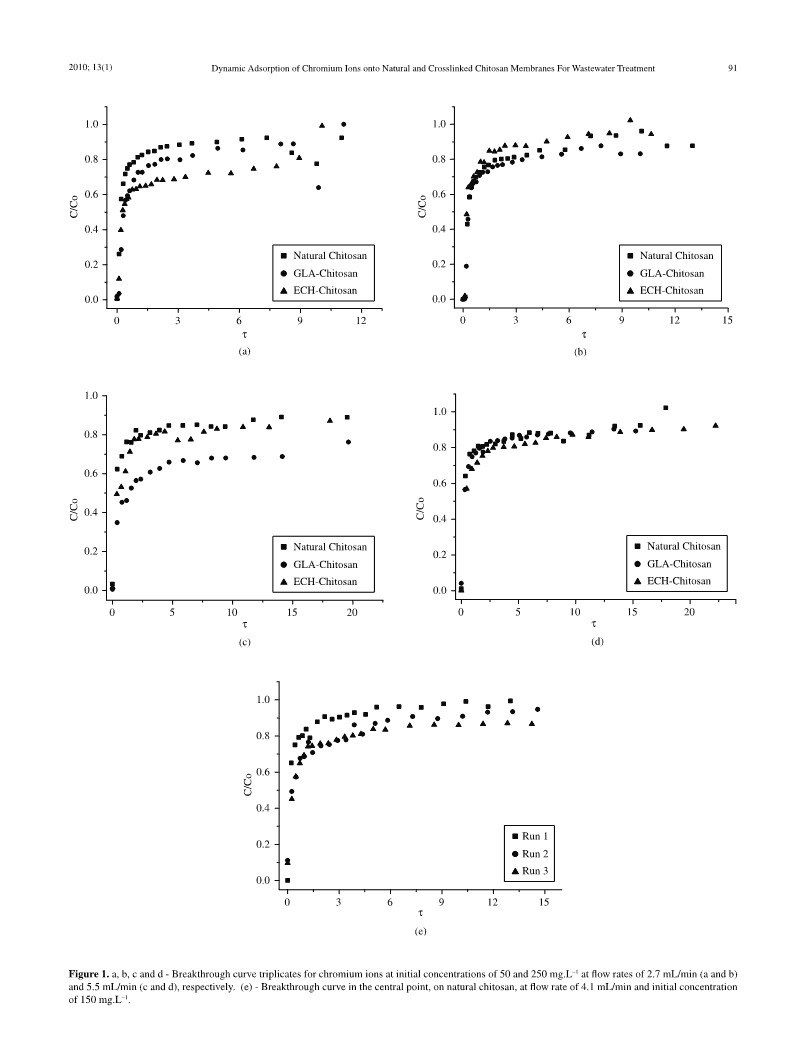
MaterialsResearch.2010;13(1):89-94©2010DynamicAdsorptionofChromiumIonsontoNaturalandCrosslinkedChitosanMembranesforWastewaterTreatmentEmersonMeneghetti,PaulaBaroni,RodrigoSilveiraVieira,MeurisGurgelCarlosdaSilva,MarisaMasumiBeppu*SchoolofChemicalEngineering,StateUniversityofCampinas,UNICAMPP.O.Box6066,13081-970Campinas-SP,BrazilReceived:October19,2009;Revised:December20,2009Waterpollutionwithheavymetalsisamatterofmajorconcernforpublichealthandalsofornaturalresourcemanagement.Thepresentstudyinvestigatedtheeffectofchemicalmodificationsonbiopolymericadsorbents(basedonchitosanmembranes)forchromiumremovalusingfixed-beddynamicadsorptiontechnique.Parameterssuchasflowrate,initialconcentrationandcrosslinkingagentswereevaluated,fromapracticalpoint-of-view,inordertooptimizetheadsorptioncapacityofnatural,glutaraldehydeandepichlorohydrincrosslinkedchitosanmembranes.Theadsorptioncapacityofnaturalandepiclorohydrin-crosslinkedchitosanmembraneswereveryclosetoeachother;however,glutaraldehyde-crosslinkedchitosanmembranespresentednearlytwicetheadsorptioncapacitycomparedtotheothermembranes,beingthemostpromisingadsorbentinsuchmass-transfersystems.Keywords:chitosan,heavymetal,chromium,adsorption1.IntroductionThecontaminationofenvironmentduetoheavymetals,evenatbranesinbatchadsorptionexperiments.Inthisstudy,wefocusedlowconcentration,isbecomingaseriousproblemandhealthconcernininvestigatingthedynamicadsorptionpropertiesusingfixed-bedduetothetypicalhightoxicityandunbiodegrabilityofsuchcontami-experiments,whicharemoreapplicabletorealindustrialprocesses.nants1.Amongthesemetals,chromiumions,whicharehighlytoxic,Statisticalanalyseswerealsoperformedinordertoevaluatetheef-areextensivelyusedinalargevarietyofindustrialactivities.fectsofinputparameters(initialconcentrationofchromium,flowChromiumisonlystableintheenvironmentwheninitstrivalentrateandcrosslinkingagent)ontheadsorptioncapacityofchitosanandthehexavalentforms.Cr(VI)ishighlytoxicandcarcinogenicmembranes.whileCr(III)presentslesstoxicityandcanbeconsideredanessen-tialmicronutrient2.However,insignificantconcentrations,Cr(III)2.MaterialsandMethodscancausefurtheradverseeffectsbecauseofitsincreasedabilitytocoordinatevariousorganiccompoundsresultingininhibitionofsome2.1.Adsorbentpreparationmetallo-enzymesystems3.Chitosansolution2.5%(w.w–1)wasprepareddissolvingchi-Theremovalofheavymetalsfromindustrialwastewatercanbeachievedusingadsorptioninafixedbedcolumn4,wheretheperform-tosanflakes(purchasedfromSigma-USA,commercialgrade)inaceticacid0.50mol.L–1solution(analyticalgrade,purchasedfromancecanbeevaluatedthroughuseofthewell-knownbreakthroughcurves.ThisconfigurationthemostinterestingfromapracticalpointLabsynth-Brazil-99.7%pure).Thesolutionswerefilteredthroughofviewsinceitallowsthetreatmentofahigheramountofwastewater28µmporosityfilterandstoredat4°C.Thepreparationofporousinacontinuousflowprocess.chitosanmembranesfollowedtheproceduredescribedbyBeppuandSantana12.ThesolutionwasspreadonaPetridish,whichwaskeptTheadsorptioncapacityofseverallow-costadsorbentshasbeeninvestigated,mainlyusingbiopolymers,suchaschitosan,whichat60°Cuntilreachingareductionof50%ofitsinitialweight.ThemembraneswereimmersedinasolutionofNaOH(1.0mol.L-1)forareobtainedfromrenewablesourcesandalsoadsorbmetallicionsselectively5-8.Itschelatingpropertiesareattributedtotheaminoand24hourstoneutralizeaminogroupsandfinallytheywerewashedhydroxylgroupspresentinchitosanchain,whichactaschelationexhaustivelywithdistilledwateruntilallalkaliwasremoved(control-sitesforseveralmetals9-10.ledbymeasuringthepHoftherinsingwater,thatshouldbeneutral)CrosslinkingreactionswithchitosanareperformedbyusingandstoredinMilli-Qwater.specificbifunctionalchemicalsthatreactwithdeterminedgroupsof2.2.Chemicalmodificationsofmembranesthebiopolymer.Thepurposedofthiskindofchemicalmodificationistoavoidchitosandissolutioninacidicsolutions,thusimproving•Crosslinkingwithepichlorohydrin–3.0gofnaturalchitosanthebiopolymermechanicalresistanceandenhancingitsadsorptionmembraneswereimmersedin50mLof0.01mol.L–1ofepichlo-capacity.Glutaraldehydeandepichlorohydrinaretwoimportantrohydrinsolution,preparedina0.067mol.L–1NaOHsolutionchemicalsusedwiththisintention.Theformerreactspreferablywithandwerekeptat40°Cundercontinuousagitationfor2hours.aminogroupswhilethelatterreactswithhydroxylgroups.ThemembraneswerethenwashedandstoredinMilli-QwaterInapreviousstudy11weinvestigatedtheeffectofcrosslinkingat4°C13.Themolarratioofepichlorohydrin/NHwas0.02.2reactioninthechromiumadsorptionpropertiesonchitosanmem-•Crosslinkingwithglutaraldehyde–3.0gofnaturalchitosan*e-mail:beppu@feq.unicamp.brn90Meneghettietal.MaterialsResearchTable1.Propertiesofnaturalandcrosslinkedchitosanmembranes15.AspectDeacetilationdegree(%)Width(mm)Watercontent(%)Porosity(%)NaturalChitosanOpaque81.01.83±0.0793.974.7GLA-ChitosanOpaque-1.93±0.1291.543.9ECH-ChitosanOpaque68.71.94±0.0593.150.7TheadsorbedamountofchromiumionsQ(mgmetal.g-1ad-Table2.Experimentalconditionsforeachfactor.sorbent)isobtainedthroughanintegralmassbalance,asgivenbyLevelsInitialconcentration(mg.L–1)Flowrate(mL/min)Equation1.+2505.5VFCentralPoint1504.1CViF()−−VCM∫dV-502.7V(1)MQ=Wmembraneswereimmersedin50mLof0.75%w.w–1glutar-inwhichtheintegralrepresentstheareabelowtheadsorptioncurve,aldehydesolutionandwerekeptundercontinuousagitationobtainedthroughnumericalintegrationusingORIGINsoftwareVF,for2hours.ThemembraneswerewashedandstoredinMilli-(L)correspondstothefeedvolume,whereC(t),timeconcentration[14](mg.L–1),Citheinitialconcentration(mg.L–1)intheeffluentsolution,Qwaterat4°C.Themolarratioglularaldehyde/NHwas25.0.VM(L)isthedeadvolumeofthesystemandWistheweightwetorTheratioofcrosslinkingagentandaminogroupscanhaveinflu-dryadsorbent(g).Thedimensionlessconcentration(C*)wasplottedagainsttheenceinthechromiumadsorptionproperties,butitwasnotquanti-tivelyinvestigatedinthisstudy.Thedifferentmolarratiovaluesofdimensionlesstimeparameter(τ),inordertocomparethedifferentglutaraldehyde/aminogroupsandepichlorohydrin/aminogroupsconcentrationsandadsorbentsused,andwerecalculatedaccordingwerechosenbasedintheliterature13-14asindicatedtomaximizethetothefollowingEquations2and3:Cr(VI)adsorptioncapacity.*Ct()Thedeacetilationdegreeofchitosanwasmeasuredbypoten-C=(2)Citiometrictitration;thethicknessofmembranesweremeasurebyaMitutoyomicrometer;watercontentwasobtainedbymassbalanceεcolvtsupτ=(3)afterdryingtheadsorbentwithinanovenuntilconstantmasswasLreached;andporositywasmeasuredbyusingagravimetricmethodaswhereL(cm)andεcolisthelengthandporosityofthecolumn,explainedinotherpreviousstudiesfromthegroup15.Table1depictsrespectively,vsup(cm/min)thesuperficialvelocityintheentrancetheseproperties15fornaturalandglutaraldehydeandepichlorohydrin-ofbed,tistheruntimeandτisthedimensionlesstimeparameter,crosslinkedchitosan.Thewatercontentisparticularlyimportanttowhichcorrespondsphysicallytothenumberofdisplacements,thecalculatethechromiumadsorptionindrybaseofbiopolymer.ratiobetweenthetotalvolumeoffluidfeedandvoidvolumeinthecolumn.Theporosityofthecolumnwasdeterminedbygravimetric2.3.Adsorptionexperimentsmethod,byknowingthedensityofadsorbentandtheadsorbentandChromiumsolutionswerepreparedusingpotassiumdichromatecolumngeometry.Thesuperficialvelocityiscalculateddividingthe(analyticalgrade,KCrO-Sigma-USA-99,9%),at50,150andvolumetricflowratebythecross-sectionalflowarea.227250mg.L-1.Theseconcentrationswerechosenbasedinaprevious2.4.Factorialplanstudy11,whichdeterminedthemaximumadsorbedamountsbystaticexperiments.Afactorialplanof3×2×2wasusedinthepresentstudy,inToperformthedynamictests,aplasticcolumn(height=20cm,ordertostudytheinfluenceofthefollowingparameters:initialdiameter=1.4cm)waspackedwithchitosanmembranepiecesofconcentrationofchromiumions,flowrateandcrosslinkingagent1cm2.Thisadsorbentgeometrywasperformedinordertoallow(varyinginthreelevels-naturalchitosan,chitosancrosslinkedwithcomparisonswithpreviousstaticexperimentresults11.Solutionepichlorhydrinorglutaraldehyde)onadsorptioncapacity.TriplicateswaspumpedbyusingaMasterflexperistalticpump,takingcaretoofthecentralpointbetweenthemaximumandminimumflowrateandnotformairbubblesinthesystem,whichcouldinducepreferentialconcentrationswereperformed,inordertodeterminetheerrorandthereproducibilitylevelofexperiments,assumingthehomoscedasticityflowpathsthroughthebed.Thesystemwasfirststabilizedusingaofthesystem16.Table2showstheexperimentalconditionsforeachcontinuousflowofMilli-Qwaterfor24hours.Thechromiumsolu-experiment,basedontheexperimentaldesign.ThisTableshowsthetionwaspumpedupward,atpH=6.0,andsampleswerecollectedhighestandlowestvaluesforeachparameterandthecentralpoint.withinspecifiedtimeintervalsandthevaluesfortotalchromiumThelevelofflowrateswerechosenbasedinpreviousstudies,obtainedconcentrationweredetermined.Totalchromiumconcentrationwasusingstaticadsorptionexperiments,whichshowedthattheadsorptiondeterminedbyatomicabsorptionspectrometry(PerkinElmerAnalystkineticsofchromiumionsonchitosanmembranesareveryslow.A100)inair-acetyleneflame,basedontheradiationofchromiumtotalof15experimentaltestswereperformed,includingthecentralatomsat357.9nm).pointtriplicates,whichwereperformedusingnaturalchitosan.TheeffectofpHonchromiumadsorptionusingchitosanbystaticexperimentswasanalyzedandthemaximumadsorbedamount3.ResultsandDiscussionsoccurredatpH=6.0,comparedwithpH=2.0,onepichlorohydrin-crosslinkedchitosan11.Therefore,inthisstudythepH=6.0,duetheInallexperiments,thepHwaskeptconstantandwasequaltohighestadsorptioncapacity,wasusedtoevaluatetheaffinityand6.0(adjusted,usingNaOHsolution(0.1mol.L–1).InthispHrange,behaviorofchromiumionsindynamicprocess.itisnotprobablethatthereductionofchromateionscantakeplace.n2010;13(1)DynamicAdsorptionofChromiumIonsontoNaturalandCrosslinkedChitosanMembranesForWastewaterTreatment91Figure1.a,b,candd-Breakthroughcurvetriplicatesforchromiumionsatinitialconcentrationsof50and250mg.L–1atflowratesof2.7mL/min(aandb)and5.5mL/min(candd),respectively.(e)-Breakthroughcurveinthecentralpoint,onnaturalchitosan,atflowrateof4.1mL/minandinitialconcentrationof150mg.L–1.n92Meneghettietal.MaterialsResearchDambiesetal.17reportedthattheentireCrboundtoglutaraldehydeahighinfluenceintheadsorptionprocess,showingthattheexternalcrosslinkedchitosanbeadsatpH4wasnon-enzymaticallyreduceddiffusionisthemostratelimiting.toCr(III)whileonly60%oftheCrboundtonativechitosanbeadsComparingthebreakthroughcurvesfromFigures1(a)and1(b),wasinthetrivalentform.Bodduetal.18alsoreportedthataboutwheretheflowratewaskeptconstantandequalto2.7mL/minand67%oftheCrboundtoacompositechitosanbiosorbent,preparedthesolutionconcentrationwasincreasedfrom50to250mg.L–1,abycoatingtheceramicsubstratewithchitosangel,wasreducedtochangeinthecurve’sshapecanbeobserved.Then,byincreasingtheCr(III)atpH4.Alltheotherexperimentalconditionsweremaintainedsolutionconcentrationfrom50to250mg.L–1,theadsorptionkineticsasconstantaspossibleduringthetests(equipments,incidentlightchanged,oncetheequilibriumwasreachedfasterinthelattercase.andmethodsforpreparingsolution).ThebreakthroughcurvesareThesameresultisobservedwhencomparingthebreakthroughcurvesshowninFigures1(a)to(e)onchitosanmembranes.TheFiguresfromFigures1(c)with1(d),wheretheflowrateswere5.5mL/min.1(a),(b),(c)and(d)depictthebreakthroughcurvesforchromiumThecurvesfromthetriplicatessuggestareproducibilitythatcanbeionsatinitialconcentrationsof50and250mg.L–1andatflowratesconsideredsatisfactory.Theadsorbedamountofchromiumionsinof2.7mL/min(aandb)and5.5mL/min(candd),respectively.TheeachexperimentalrunisshowedinTable3.Chromiumionsadsorp-Figure1(e)showsthebreakthroughcurve,onnaturalchitosan,intiononchitosanhasslowkineticratesandinsomeexperimentsthethecentralpointaflowrateof4.1mL/minandaninitialconcentra-equilibriumwasnotreached.Thisfactexplainsthedifferenceinthetionof150mg.L–1.adsorbedamountatdifferentflowrate,forexampletonaturalchitosanFromtheFigure1itispossibletoobservethatthebreakthroughatinitialconcentrationof250mg.L–1.occurredalmostimmediatelyinthebeginningoftheprocesses,dueThehighestvaluesofadsorbedmetalquantitywerethosefoundtothelowkineticrateofchromiumionsonchitosanmembranes11.forchitosancrosslinkedwithglutaraldehyde(nearlytwicethevalueThisphenomenonismainlyinfluencedbychitosanandchromiumfoundforothermembranes).Evenwiththecrosslinkingprocessionaffinityandthemembranegeometryused,sinceitssizewaslargeinducedwithglutaraldehyde,anincreaseinadsorptioncapacitycomparedtothecolumndimensions.Theintentionofthisworkwasoccurred.SimilarresultswereobtainedbyVieiraandBeppu15,19,20,indeedtoanalyzetheaffinitybetweenadsorbentandadsorbateinawhostudiedmercuryadsorptiononchitosanusingstaticanddynamicdynamicprocess,whereasthestaticprocesswaspreviouslystudied,methods.Machadoetal.21showsanincreaseincopperadsorptiongivingsatisfactoryresults11.Theconditionsofexperimentswereafterglutaradehydecrosslinkingreaction,andthisfactisattributedtochosenaccordingtotheaveragerequirementsfoundinsuchadsorp-theamountoffreeaminogroups,astherewouldbelessprotonatedtivesystems:theconcentrationofsolutiontobetreatedwassetbyaminogroupsinthecrosslinkedmatrices.Thesameeffectcouldbetheamountofpollutants;theamountofchitosanplacedwithinthecausingtheobservedresultsforchromiumions.columnwasmostlylimitedbychitosandensityand,hence,bytheAccordingtoHsienandRorrer14anincreaseinadsorbingcati-volumetofillanadsorptioncolumn;andtheflowratewaslimitedonsaftercrosslinkingprocessisexplainedbytheincreaseinspacebythevolumeofeffluenttobetreated.amongchitosanchains,whichisresponsibleforanimprovementinFromapracticalpoint-of-view,ifthebreakthoughoccursverytheaccessibilityofthemetallicionstoaminogroups.Inthisway,quickly,thebestconfigurationtousethistechnologywillprobablyanenhancementintheadsorptioncapacitycanbeinterpretedasbetheapplicationofmanycolumnsinaparallelfeature.aresultofincreasingtheaccessibilityformetallicionsduetotheComparingthebreakthroughcurvesofFigure1(a)withthatpartialdestructionofthecrystallinestructure22.AccordingtoKuritaof1(c),wheretheconcentrationswere50mg.L–1,itispossibletoetal.23thecrystallinityhasafundamentalroleintheaccessibilityobservethat,whiletheflowrateincreasedfrom2.7to5.5mL/min,ofadsorbentgroupstometallicionsandmanystudieshavebeenthevaluesofC(t)/Cdidnotchangesignificantlyforeachkindoftryingtodemonstratethatwhencrystallinityisreduced,anincreaseiadsorbent.Similarobservationscanbegatheredwhencomparinginadsorptivecapacityispromoted.BeppuandSantana12showedtheplotsofFigures1(b)and1(d),wheretheconcentrationswerethroughX-raydiffractionthatadecreaseincrystallinityoccurs250mg.L–1.ItindicatesthatthevariationinflowratedidnotpresentonmembranescrosslinkedwithglutaraldehydecomparedtotheTable3.Adsorbedamountofchromiumionsineachexperimentalrun.FlowrateInitialconcentrationAdsorbedamountQAdsorbedamountQwetdry(mL/min)(mg.L–1)(mg.g–1wetchitosan)(mg.g–1drychitosan)NaturalChitosan2.70500.548.92503.0550.05.50500.437.02501.7729.1GLA-Chitosan2.70500.829.72504.1148.35.50501.3215.52502.8333.4ECH-Chitosan2.70500.9113.22501.9728.55.50500.689.92501.8126.3Triplicate–Natural4.101500.498.1Chitosan1.0216.71.2720.9n2010;13(1)DynamicAdsorptionofChromiumIonsontoNaturalandCrosslinkedChitosanMembranesForWastewaterTreatment93naturalone.MonteiroandAiroldi24studiedtheadsorptioncapacityTable4depictsthestatisticaldatarelatedtoadjustmentofre-sultswithR2adjustedparameters.Table4depictstheeffectsthatofchitosanforCu(II)ionsanditwasobservedthatcopperremainsadsorbedontochitosanevenaftercrosslinkingwithglutaraldehyde.eachfactorcausesinthesystem,i.e.theresultantquantitativeeffectTheauthorssupposedthatthenewstructureformedbycrosslinkingduetoamodificationofafactor.Table4andtheParetodiagraminreaction,iminobounds,isabletoadsorbcopperions.Figure2(a)indicatethatthemostimportanteffectintheamountTheadsorptionresultsobtainedfornaturalandepichlorhydrin-ofmetaladsorbedwastheinitialsolutionconcentration(A),fol-lowedbyflowrate(B)andtheinteractionbetweenthesetwoeffectscrosslinkedchitosanwerequitesimilar,whichindicatesthepossibility(AB).Thepositivevalueof1.667oftheconcentrationeffect(C)oftheadsorptionofmetallicionscantakeplaceonaminogroups,iindicatesthatanincreaseintheconcentrationcausesanincreaseinwhichareavailable,andinlessproportiononhydroxylgroups,whichthechromiumadsorption.Similarly,anegativevalueinsomefac-canbeunavailablebycrosslinkingreaction.Theseresultsareinac-torsindicatesthatwhenincreasingthevalueofthisfactortheeffectcordancewithBaronietal.11andVieiraandBeppu15whoinvestigatedisdecreased.Theconcentrationfactorpresentedtrustablep-valuestheadsorptionmechanismofchromiumandmercuryionsonchitosan(0.052),whileflowrateandtheinteractionbetweenthesetwoeffectsusingFTIR-ATR.Theauthorsdemonstratedthatthemetalinterac-showedhighp-values.tionismainlyduetothecharacteristicofthechitosan,suchas,iftheAnalyzingtheplotfromFigure2(A),itwaspossibletosaythatmaterialiscrosslinkedornotandthekindofcroslinkingagent.theconcentrationeffecthasthebiggestabsolutevaluesbeingtheTheglutaraldehyde-crosslinkedchitosanprovidesthebestquanti-maineffectontheanalyzedresponse.tativeresultsforchromiumadsorption,butthenaturalchitosanoffersThecoefficientvaluesofTable4givetheEquation4whichadvantageofnotneedingfurtherchemicaltransformationandcanberepresentsthequantityofadsorbedmetalasafunctionoftheflowsuitableforapplicationinrealsituations.rateandtheinitialconcentrationfromchromiumsolution,usingTheevaluationofchromiumdesorptionfromchitosanisverynaturalchitosan.importanttoverifythepossibilityofitsreuse.Somedesorptionexperi-Q=1.3078+0.8338.Ci–0.2888.W–0.2438.mentswereperformedinstaticprocesses,suchaspreviouslydescribedbyBaronietal.11anddesorptionof48.6%forchromiumtotalionsCi.W–0.3819.(Pt.C(t))(4)atpH=6.0wasobservedonnaturalchitosan.Inaddition,chitosanwhereP.C(t)isthecentralpointmean.membranesweresubmittedforthreecyclesofadsorption/desorption,R2(adjusted)of75.87%showsthatthelinearmodelofthefac-indicatingafavorablepossibilityofchitosanreutilization11.torialexplainspoorlythebehaviorofchromiumionadsorptiononByusingMinitab®software,Paretodiagramswereobtainedchitosanbiopolymer.Onereasonforthatcanbetheattributedtoaforfactorsstudiedintheexperimentalplan(Figures2a,bandc)possiblenon-linearityoftheresponse-factorrelationship.fornaturalchitosan.AnyvaluethatgoesbeyondtheredlineintheTheParetodiagramsfromFigures2(b)and2(c)alsoindicatediagramhasasignificancelevelof0.05,representingaconfidencethattheinitialconcentrationofchromiumsolutionpresentsthemainof95%.Thep-valuecanalsobeseeninthesamefigures.effectamongthestudiedfactors.ThisconcentrationisusuallyafixedFigure2.(a),(b)and(c)–Paretodiagramsfornatural,epichlorohydrin-crosslinkedandglutaraldehyde-crosslinkedchitosanmembranes,respectively.A=con-centration,B=flowrate,AB=interactionbetweenAandB.n94Meneghettietal.MaterialsResearchTable4.Statisticaladjustmentdatafornaturalchitosan.6.CriniG,BadotPM.Applicationofchitosan,anaturalaminopolysaccharide,fordyeremovalfromaqueoussolutionsbyadsorptionprocessesFactorEffectCoefficientpusingbatchstudies:Areviewofrecentliterature.ProgressinPolymerConstant*1.30780.022Science.2008;33(4):399‑447.C(mg.L–1)1.66750.83380.052i7.TrimukheKD,VarmaAJ.ComplexationofheavymetalsbycrosslinkedFlowrate(mL/min)–0.5775–0.28880.283chitinanditsdeacetylatedderivatives.CarbohydratePolymers.2008;C(mg.L–1)*Flowrate(mL/min)–0.4875–0.24380.34471(1):66‑73.iPt*C(t)*–0.38190.3358.GerenteC,LeeVKC,CloirecPL,McKAYG.ApplicationofChitosanforR2(adjusted)=75.87%theRemovalofMetalsFromWastewatersbyAdsorption—MechanismsandModelsReview.CriticalReviewsinEnvironmentalScienceandTechnology.2007;37(1):41‑127.conditionoftheindustrialeffluenttobetreatedandusuallycannotbechanged,mainlyiftherequiredconditionisamoreconcentrated9.BassiR,PrasherSO,SimpsonBK.Removalofselectedmetalionsfromaqueoussolutionsusingchitosanflakes.SeparationScienceTechnology.solution.Hence,onecouldsaythattheuseofchitosanasadsorbent2000;35(4):547-560.islimitedtoanon-controllablefactorsuchastheinitialconcentrationofchromium;however,manycyclesofadsorptioncouldbeapplied10.GuibalE.Interactionsofmetalionswithchitosan-basedsorbents:Atoresultinagivenrequiredpurification.Review.SeparationPurificationTechnology.2004;38(1):43-74.Furtheranalysesarebeingperformedinthegroupinorderto11.BaroniP,VieiraRS,MeneghettiE,daSilvaMGC,BeppuMM.Evaluationproposeamodelofchromiumionadsorptionandtoelucidatetheofbatchadsorptionofchromiumionsonnaturalandcrosslinkedchitosanspeciesthatareatlastbeingadsorbedontochitosansurface.membranes.JournalofHazardousMaterials.2008;152(3):1155-1163.12.BeppuMM,SantanaCC.PAAinfluenceonchitosanmembrane4.Conclusionscalcification.MaterialsScienceEngineeringC.2003;23(5):651-658.13.WeiYC,HudsonSM,MayerJM,KaplanDL.ThecrosslinkingofchitosanDynamicadsorptioninfixedbedexperimentspresentedsatisfac-fibers.JournalofPolymerSciencePartA:PolymerChemistry.1992;toryresultsregardingtotheadsorptioncapacityofchromium.The30(10):2187-2193.slowkineticrateofchromiumionsresultedinabreakthoughvery14.HsienTY,RorrerGL.EffectsofAcetylationandCrosslinkingonThequickly;howeveritwaspossibletoanalyzetheeffectofflowrate,MaterialPropertiesandCadmiumIonAdsorptionCapacityofPorousinitialconcentrationandcrosslinkingreactiononadsorbedamount.ChitosanBeads.SeparationScienceTechnology.1995;30(12):2455-75.Initialchromiumsolutionconcentrationisthemainfactorfrom15.VieiraRS,BeppuMM.Interactionofnaturalandcrosslinkedchitosantheonesthatwereconsidered,asitinfluenceschitosanadsorptionca-membraneswithHg(II)ions.ColloidsSurfaceA-Physicochemicalandpacity,establishingapositiverelationshipbetweenthesevariables.EngineeringAspects.2006;79(1‑3):196‑207.Thecrosslinkingperformedonchitosanwithglutaraldehydewas16.BoxGEP,HunterWG,HunterJS.Statisticsforexperimenters:anthecondition,amongotherchemicaltreatments,thatpresentedtheintroductiontodesign,dataanalysis,andmodelbuilding.NewYork:J.highestchromiumuptakingcapacity.Althoughbeingsatisfactory,Wiley;1978.theothertwoformsofchitosanpresentedsimilaradsorptioncapac-17.DambiesL,GuimonC,YiacoumiS,GuibalE.Characterizationofmetality,lowerthanthecapacityexhibitedbyglutaraldehyde-crosslinkedioninteractionswithchitosanbyX-rayphotoelectronspectroscopy.chitosan.ColloidsSurfaceA-PhysicochemicalandEngineeringAspects.2001;177(2-3):203-214.Acknowledgements18.BodduVM,AbburiK,TalbottJL,SmithED.RemovalofhexavalentTheauthorsthankFAPESPandCNPqfortheirfinancialsupportchromiumfromwastewaterusinganewcompositechitosanbiosorbent.andscholarship.EnvironmentalScienceTechnology.2003;37(19):4449-4456.19.VieiraRS,BeppuMM.MercuryionrecoveryusingnaturalandReferencescrosslinkedchitosanmembranes.Adsorption,2005;11(1):731-736.20.VieiraRS,BeppuMM.Dynamicandstaticadsorptionanddesorptionof1.RapsomanikisS,CraigPJ.SpeciationofMercuryandMethylmercuryHg(II)ionsonchitosanmembranesandspheres.WaterResearch.2006;CompoundsinAqueousSamplesbyChromatography-AtomicAbsorption40(8):1726-1734.SpectrometryafterEthylationwithSodiumTetraethylborate.AnalyticaChimicaActa.1991;248(2):563-567.21.MachadoMO,LopesECN,SousaKS,AiroldiC.Theeffectivenessof2.NieboerE,JusysAA.Biologicchemistryofchromium.In:Nriagu,JOtheprotectedaminogrouponcrosslinkedchitosansforcopperremovalandNieboerE,editor.ChromiuminNaturalandHumanEnvironments.andthethermodynamicsofinteractionatthesolid/liquidinterface.NewYork:WileyInterscience;1988.CarbohydratePolymers.2009;77(4):760‑766.3.KotasJ,StasickaS.ChromiumOccurrenceintheenvironmentandmethods22.KoyamaY,TaniguchiA,HuangCP,BlakenshipDW.Studiesonchitin.X.ofitsspeciation.EnvironmentalPollution.2000;107(3):263‑283.Homogeneouscross-linkingofchitosanforenhancedcupricionadsorption.JournalofAppliedPolymerScience.1986;31(6):1951-1954.4.DutaFP,FrancaFP,CostaACA.Theperformanceofacontinuoussystemforbiosorptionanddesorptionofzinc,cadmium,manganeseandcopper23.KuritaK,SannanT,Iwakura,Y.StudiesonChitin.VI.BindingofMetalbytheseaweedSargassumsp.EuropeanJournalofMineralProcessingCations.JournalofAppliedPolymerScience.1979;23(2):511-515.EnvironmentalProtection.2002;2(3):131-140.24.MonteiroJr.OAC,AiroldiC.Somestudiesofcrosslinking-glutaraldehyde5.BaileySE,OlinTJ,BrickaRM,AdrianDD.Areviewofpotentiallylow-costinteractioninahomogeneoussystem.InternationalJournalofBiologicalsorbentsforheavymetals.WaterResearch.1999;33(11):2469-2479.Macromolecules.1999;26(2-3):119-128.