Controlled synthesis and wastewater treatment ofAg2OTiO2 modified chitosan-based photocatalytic控制合成与 11页
- 1.41 MB
- 2022-04-26 发布
Controlled synthesis and wastewater treatment ofAg2OTiO2 modified chitosan-based photocatalytic控制合成与

- 1、本文档由用户上传,淘文库整理发布,可阅读全部内容。
- 2、本文档内容版权归属内容提供方,所产生的收益全部归内容提供方所有。如果您对本文有版权争议,请立即联系网站客服。
- 3、本文档由用户上传,本站不保证质量和数量令人满意,可能有诸多瑕疵,付费之前,请仔细阅读内容确认后进行付费下载。
- 网站客服QQ:403074932
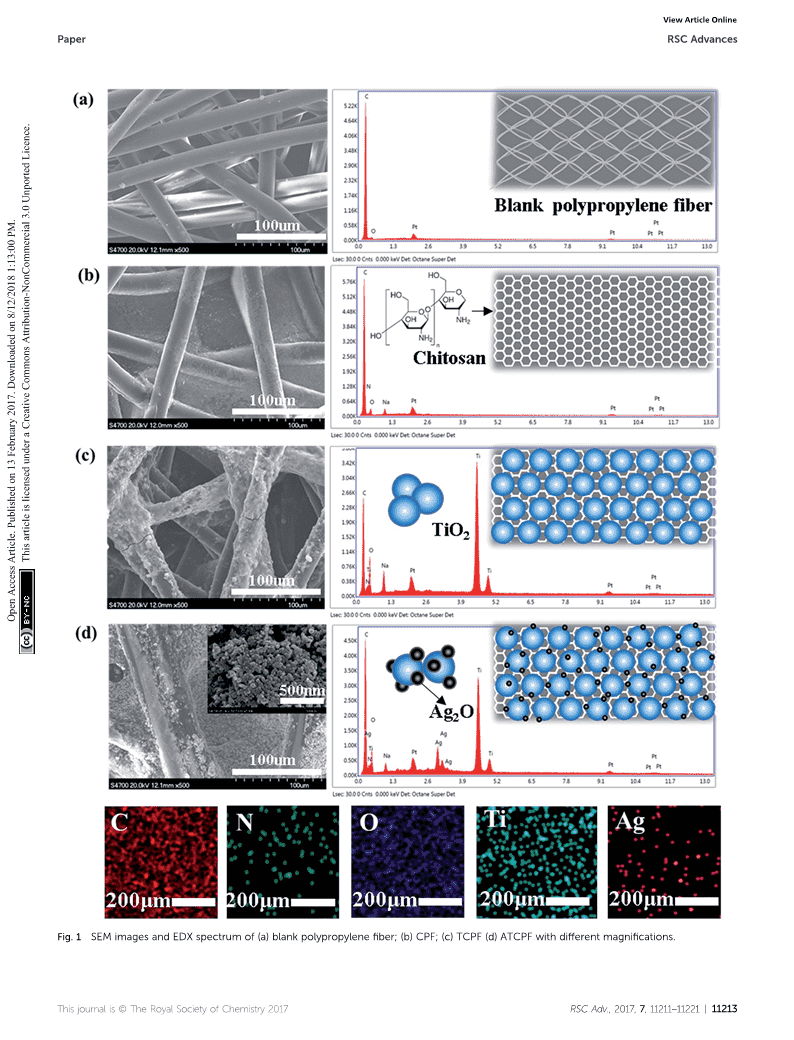
RSCAdvancesViewArticleOnlinePAPERViewJournal|ViewIssueControlledsynthesisandwastewatertreatmentofAg2O/TiO2modifiedchitosan-basedphotocatalyticCitethis:RSCAdv.,2017,7,11211filmYilinZhao,ChengranTao,GangXiaoandHaijiaSu*AnovelAg2O/TiO2-modifiedchitosan-basedphotocatalyticfilmwithhighadsorptionandphotocatalyticactivitywassynthesizedundersimulatedsolarirradiation,baseduponthecouplingofthesynergisticcatalytictechniqueofnanoAg2O/TiO2andmembraneseparation.XRD,XPS,FESEM,andTEMcharacterizationresultsillustratedthattheAg2Onanoparticleswithasmallsizeof3–5nmdepositedonthesurfaceofTiO2.Subsequently,Ag2O–TiO2nanoheterojunctions,aswholegraft-structures,werecoatedonachitosan-basedpolypropylenefilm.Theas-synthesizedphotocatalyticfilmwithawideReceived24thNovember2016visible-lightadsorptionbandandlowerbandgapof2.4eVenhancedthedegradationabilityforbothAccepted6thFebruary2017ampicillinandmethylorangeowingtothesynergisticeffectofAgO2/TiO2nanoheterojunctions.CreativeCommonsAttribution-NonCommercial3.0UnportedLicence.DOI:10.1039/c6ra27295aMoreover,thephotocatalyticfilmdisplayedexcellentrecyclingpropertiesforthedegradationofmethylrsc.li/rsc-advancesorangebybeingreusedfor5timeswithoutlosingitsphotocatalyticactivity.relativelylow-pricedandhighefficiency.16AgOnanoparticles,1.Introduction2abrownpowderwithanarrowbandgapof1.3eV,areefficientPersistentorganicpollutants(POPs)arecausingconcernedelectronabsorbingagentsunderUVlightirradiationandsensitizerstoturnUVlightresponseintothevisibleregion.17–19amongthescienticcommunityduetotheirpersistenceintheenvironment.1ThephotocatalyticprocessofnanoTiOhasBesides,Ag2Ohasbeenusedinvariouselds,asamildoxidant,2Thisarticleislicensedunderawatercleaningagent,andcatalyst.20TheadvantagesofAgOshowntremendouspotentialasahigh-efficiency,low-cost,high2physical-chemicalstableandenvironmentallyfriendlytech-impelanovelstrategyofcombiningAg2OandTiO2forsuperiornologytodegradePOPsinwastewater.2,3Underultraviolet(UV)photocatalystsinthevisiblelightregion.OpenAccessArticle.Publishedon13February2017.Downloadedon8/12/20181:13:00PM.lightirradiation,electronsareemittedfromTiO2inducingAnothermaindrawbackofusingnanosizedTiO2isitscomplicatedsubsequentseparationfromwater.21Tohandleelectron–holepairformation,andsubsequentchargesepara-thisproblem,variousmaterials,suchasglass,22stainlesssteeltioninducestheproductionofhighlyreactiveoxygenspeciesmesh,23ber,24silicagel25andpolymericmaterials,26havebeenthatcancompletelymineralizePOPs.However,TiO2onlyrespondstoUVlightwithawavelengthbelow387nm.4ThisexploredasaTiO2supportforthephotodegradationofdrawbacklimitsthepossibilityofemployingsunlightasalightcontaminantsinwastewater.Couplingnano-TiO2withorganicsourcebecauseUVlightoccupiesfewerproportions(4%)ofthepolymerstofabricatemembrane/globularcompositesisansun'sradiationenergythanthatofvisiblelight(43%).Inefficientwayforimmobilization.Chitosan(CS)isthesecondaddition,highrecombinationrates,about10nsbetweenelec-mostabundantnaturalpolymerobtainedfromthecellwallsoftronandholepairs,reducethequantumyieldofasingleTiO2fungiandtheshellsofcrust.CShasdrawnagreatattentionforsemiconductor.5itsexcellentproperties,suchasbloodcompatibility,microbialdegradationandenvironmentalsecurity.27UsingchitosanasInordertoimprovethephotocatalyticactivityandvisiblelightutilizationofTiO2,numerousstudies,pertainingtotheacarrierforTiO2,chitosan-supportedTiO2(CS–TiO2)adsorbentsurfacemodicationofTiO2nanoparticleswithdepositingwaspreparedandexhibitedmultifunctionalperformancefornoblemetalNPs(Au,Ag,Pt,Pd,etc.),6–10andcouplingwithincreasingtheadsorptioncapacityofheavymetalsandsemiconductors,suchasCdS,11AgO,12VO,13WO14andenhancingtheadsorption–photocatalyticprocessoforganic225315pollutants.28Asaresult,twoformsofCS–TiOcompositeswereBi2O3,havebeencarriedoutandobtainedexcellentachieve-2ments.Comparedwithothermetallics,AgandAg-basedoxidesprepared:membraneandbeads.Althoughglobularresinscanarethemostsuitableforpracticalapplicationsbecauseoftheireffectivelydegradecontaminants,thephotocatalyticefficiencyofCS–TiO2beadsisusuallylowerthanthatofmembranesystemsduetothelimitedcontactareawithcontaminants.ItisBeijingKeyLaboratoryofBioprocess,BeijingUniversityofChemicalTechnology,highlydesirabletodevelopmembraneCS–TiO2compositesBeijing100029,PRChina.E-mail:suhj@mail.buct.edu.cn;Fax:+86-10-64414268;Tel:+86-10-64452756withabetterphotocatalyticability.Thisjournalis©TheRoyalSocietyofChemistry2017RSCAdv.,2017,7,11211–11221|11211nViewArticleOnlineRSCAdvancesPaperBasedonthepotentialsynergisticeffectsofAg2OandTiO2,photocatalyticlm(TCPF),Ag2O/TiO2nanocomposites(AT)anewmultilayerphotocatalytic-membranewasfabricated,werealsopreparedascontrol.consistingofaAg2O/TiO2layerstackedonachitosansub-layerimmobilizedontoapolypropylenebersubstrate.FESEM,EDX,2.3CharacterizationXRD,HRTEM,XPS,andUV-visspectroscopywereusedtoThemorphologyofATCPFwasobservedbyaFE-SEM(HitachiS-characterizethelms.ThephotocatalyticperformanceofAg2O/4700,Tokyo,Japan)andaHRTEM(JOELJEM3010,Tokyo,TiO2-modiedchitosan-basedphotocatalyticlm(ATCPF)wasJapan).ThecrystallinephasecontainedinTiO2andAg2O/TiO2analyzedbythedegradationofampicillin(AMP)andmethylwasobservedbyXRDpatternsinaRigakuD/Max2400diffrac-orange(MO)withvisiblelightirradiation.Chitosan-supportedtionmeter(Tokyo,Japan).XPSwasrecordedwiththeESCALABTiO2photocatalyticlm(CTPF)wasalsopreparedandits250system(ThermoFisherScientic,USA).UV-visdiffusephotocatalyticperformancewasevaluatedwithtwomodelreectancespectrawereconductedonaUV-visspectropho-pollutantsasreference.Thereusepropertyoftheobtainedtometer(UV-3150,Shimadzu,Japan)attachedwithintegratingATCPFwastestedforabetterunderstandingofitspotentialsphere.Allsamples(5mL)wereltratedwitha0.45mmltertocapabilityforpracticalapplications.Thepresentstudydevel-removeCODandnitrogen,subsequentlyquantiedthemajoropedanefficientphotocatalysttowardsorganicpollutantsand4++cationiccontents(TiandAgions)byanAgilent7700xICP-MSprovidednoveldesignideasforeffectivevisible-light-mediated(Agilenttechnologies,USA).nanocompositesphotocatalysts.2.4Photocatalyticactivity2.ExperimentalsectionThephotocatalyticperformanceofthesampleswasevaluated2.1Chemicalsbydegradingtwoorganiccompounds:MOandAMPPhoto-HydrophilicpolypropyleneberwaspurchasedfromHengyuechemicalexperimentswereaccomplishedinaphotocatalyticCreativeCommonsAttribution-NonCommercial3.0UnportedLicence.NonwovensCompany.Chitosan(90%degreeofdeacetylation)reactionchamberprovidedwithone150Wtungsten–halogenwasboughtfromJinanHaidebeiMarineBioengineeringCo.lampthatfacilitatedcontinuousemissionofUV-visiblelight(lLtd.NanosizedbicrystallineTiO(P25,50m2g1,average¼360–2000nm).Reactionswereperformedinacommercially2particlesize30nm)waspurchasedfromDegussa.Sodiumavailabledishwithadiameterof90mm.Inatypicalphoto-ampicillin(AMP)wasboughtfromSigmaAldrich(CAS:69-52-3).catalyticexperiment,photocatalyticlmwithadiameterof801OtherchemicalswereobtainedfromBeijingChemicalPlant,mmwasdispersedinto40mLMO(10mgL)solutionor401includingammoniumhydroxide(NH4OH),silvernitratemLAMP(20mgL)solutionwithconstantstirring.Here,each(AgNO3),methylorange(MO),aceticacid(CH3COOH),pieceofATCPForTCPwascoatedwith60mgloadingmassofepichlorohydrin(C3H5OCl)andsodiumhydroxide(NaOH).Allcatalysts(Ag2O/TiO2orTiO2).Thisarticleislicensedunderachemicalswereofanalyticalgrade,andusedwithoutfurtherTheresidualconcentrationsofMOweredetectedbyUV-vispurication.spectrophotometry(Varian,Cary100)atthewavelengthof464nm.ThedegradationofAMPwasdetectedbyHPLCanalysisOpenAccessArticle.Publishedon13February2017.Downloadedon8/12/20181:13:00PM.withaC18(5mm,4.6mm150mm)columnatthewavelength2.2Samplepreparationof220nm.ThedegradationrateofMOandAMPcouldbe2.2.1.Synthesisofchitosan/polypropylenelm(CPF).(i)calculatedbyeqn(1):Thepolypropyleneberwasshakenina2.5%NaOHaqueousC0Ctsolutionfor24htocompletebasication,followedbyadryingDegradationrateð%Þ¼100%(1)C0processatroomtemperature.(ii)0.1gCSwasdissolvedinto101mL2.5%CH3COOHliquidanddispersedwith100Wultra-whereC0andCtaretheinitialandnalconcentration(mgL),soundfor15min.Then,1mLepichlorohydrin(cross-linkingrespectively.agent)wasaddedandstirredconstantlyfor4hat25C.(iii)TheantibacterialeffectofthesampleswasanalyzedbyTheemulsionwasdrippedonthepolypropylenelmtofullyantibacterialtestsandDiskDiffusionAssaysusingthemethodintroducedbyM.J.Umapathy.6,29coverit.Bynaturallydryingthewetchitosan/polypropyleneber,CPwithasmoothsurfacewasobtained.2.2.2.SynthesisofAg2O/TiO2-modiedchitosan-based3.Resultsanddiscussionphotocatalyticlm(ATCPF).(i)0.1gTiO2nanoparticleswere3.1Characterizationofthepreparedcompositedispersedin20mLof10mMAgNO3aqueoussolution.(ii)photocatalystsNH4OHwasdrippedintotheaboveTiO2–AgNO3mixturesolu-tiontoadjustthepHbetween10and11,followedbyphoto-3.1.1.MorphologicalinvestigationwithSEM,EDSanddepositionunder365nmUVlamp(GE,20W)andvigorouslyTEM.TheFE-SEMsofthesesampleswereshowninFig.1.stirringfor2h.(iii)AerswitchingofftheUVlamp,CPFwasComparedtopristinepolypropyleneber(Fig.1a),boththeimpregnatedintothesolutionforreactinganother5hwithsurfaceandgapofblankberwerecoveredbysmoothlms,astirringrateof100rpminthedark.Finally,ATCPFwasindicatinggoodviscositystabilityandlm-formingpropertiesneutralizedandrinsedwithdeionizedwaterwhensemi-damp,ofthechitosan/aceticacidsolution(Fig.1b).TiO2nanoparticlesandthenvacuum-driedat40C.TiO-modiedchitosan-basedwereevenlycoatedonthesurfaceoftheCPaersynthesizing211212|RSCAdv.,2017,7,11211–11221Thisjournalis©TheRoyalSocietyofChemistry2017nViewArticleOnlinePaperRSCAdvancesCreativeCommonsAttribution-NonCommercial3.0UnportedLicence.ThisarticleislicensedunderaOpenAccessArticle.Publishedon13February2017.Downloadedon8/12/20181:13:00PM.Fig.1SEMimagesandEDXspectrumof(a)blankpolypropylenefiber;(b)CPF;(c)TCPF(d)ATCPFwithdifferentmagnifications.Thisjournalis©TheRoyalSocietyofChemistry2017RSCAdv.,2017,7,11211–11221|11213nViewArticleOnlineRSCAdvancesPaperTCP(Fig.1c).WhenAg2O/TiO2hadbeendepositedonCPsurfaceofTiO2withanaveragediameterof3–5nm(Fig.2a).The(Fig.1d),aroughsurfacecoatedwithhomogeneousgranulationresolvedinterplanardistanceswas0.27nm,correspondingtowasdeveloped.Withthe500magnication,obviousnano-the(111)planeofAg2O(Fig.2b).OnthebasisoftheSEMandclusterswererevealedandillustratedinFig.1d(inset),fromTEMobservation,aschematicstructureoftheATCPwasdepic-+whichtheparticlesizeofaggregatedAg2O/TiO2wasmeasuredtedinFig.2c.InthealkalineAgNO3solution,Agwasrstlyintherangeof50–500nm.ItwastheresultofnanoparticleadsorbedtothesurfaceofTiO2andthentransformedintoAg2OaggregationathighTiO2-NPscontentduringthepreparation.underUVlightillumination.ThesubsequentadditionofWiththeevencoverageofpolypropyleneberbychitosan,thechitosan/polypropyleneberprovidedanexcellent-carrierforthechitosan/polypropylenebercomposites(CP)providedaplat-nanocrystalsloading.Asaresult,Ag2O/TiO2wasdepositedonformwithhighsurfaceareaforadhesionofAg2O/TiO2.thechitosan-modiedpolypropyleneber,makingthewhiteSurfaceelementalanalysiscontainingEDXspectrumhasbeenbercolorpurple-black.OurearlierstudyofFT-IR30indicateddoneandtheresultsareshowninFig.1respectively.TCPshowsthatthehydrogenbondinginteractionwasamaindrivingforcemoredistinctelementalpeaksofTiandATCPFshowsmorefortheself-assemblybetweenTiO2withsurfacehydroxylgroupdistinctelementalpeaksofAgandTithanthatofCP.Differentandchitosanwithaminoandhydroxyl.Similarly,Ag2O-modiedcolorareasshowninFig.1dindicateC-,N-,O-,TiandAg-enrichedTiO2alsocouldeasilycombinewithchitosan/polypropyleneberareasofATCPF.Itrevealedthattheobtainedlmwascompositedviahydrogenbondinginteraction.OnthebasisoftheformationoftheelementsofC,N,O,TiandAg,andalsoshowedthemechanism,ATCPFwithagra-likestructurehadbeensynthe-homogeneousdistributionoftheAgionsonthesurface.Thesized,andshownappropriateforfollow-upapplication.peaksofCelementmainlyresultedfromthepolypropylenelm,3.1.2.Elementalcompositionsanalysis.ToprovethewhilethepeakofNelementresultedfromchitosan.TheresultssynthesisofATCPF,thecrystalstructuresandchemicalstatusdemonstratedhigh-densityTiO2andAg2Ohadbeenimmobilizedofthesesampleswereanalyzed.XRDpatternsofTCPandonthesurfaceoftheheterozygousnanocomposites.ATCPFwereshowninFig.3a.TheXRDpatternofTCPdisplayedCreativeCommonsAttribution-NonCommercial3.0UnportedLicence.InordertofurtherconrmthedistributionofbothTiO2andthatTiO2hasabicrystallinestructure,whichcanbeassignedtoAg,morphologydetailsoftheATCPFwereanalyzedbyHRTEM.anataseandrutilephases.AercoatingwithAgnanoparticles,DispersedAg2OnanoparticlesweretightlycoupledontotheATCPFshowedadditionalpeakswhencomparedwithTCPF.ThisarticleislicensedunderaOpenAccessArticle.Publishedon13February2017.Downloadedon8/12/20181:13:00PM.Fig.2(aandb)HRTEMimageand(c)schematicstructureoftheATCPF.11214|RSCAdv.,2017,7,11211–11221Thisjournalis©TheRoyalSocietyofChemistry2017nViewArticleOnlinePaperRSCAdvancesCreativeCommonsAttribution-NonCommercial3.0UnportedLicence.ThisarticleislicensedunderaOpenAccessArticle.Publishedon13February2017.Downloadedon8/12/20181:13:00PM.Fig.3(a)XRDpatternofTiO2andAg2O/TiO2scrapedofffromATCPF(A:anatase,R:rutile).(b)XPSsurveyscanspectraofpureTiO2andAg2O/TiO2;(c)XPSspectraforAg3d5/2and3d3/2;(d)XPSspectraforTiO22P3/2and2P1/2;(e)UV-visabsorptionspectraofAg2O/TiO2andTiO2;(f)theband-gapenergiesofATCPFandTCPF;(g)theenergybandstructureofAg2OandTiO2beforecontactandtheenergybandstructureofp-Ag2O/n-TiO2heterojunctionatequilibrium(Evac:vacuumlevel;EF:Fermilevel;f:workfunction;c:electronaffinity).TwoobviouspeaksforAgOweredetectedat32.8{111}(JCPDS(Fig.3d).31Itindicatedthatsilverexistedinonlyonevalence2no.41-1104)and46.14{300}(JCPDSno.42-0874),whichstate(Ag+)asAgOintheobtainedmixture.Therefore,the2demonstratesthattheAg2OnanoparticlesarecoatedonthenanoparticlesscrapedfromATCPFconrmedthatTiO2nano-surfaceofTCPbutnotinsertedinthecrystallattice.particlesweresuccessfullymodiedbyAg2ObasedonXRDandTheelementalcompositionsandchemicalstatusofTCPFXPScharacterization.andATCPFwerecharacterizedbyXPS(Fig.3b–d).Compared3.1.3.Mechanismofphotocatalyticactivity.TheUV-viswithTCP,additionalpeaksofAgwerefoundinATCPFindiffusereectancespectraforTCPandATCPFareshowninadditiontotheTi,O,andCpeaks(Fig.3b).High-resolutionFig.3e.AbroadintenseadsorptionofTCPFisintheUVregionscanoverAg3d5/2inAg2O/TiO2nanoparticlesdemonstratedbelow400nm;however,astrongandwideadsorptionofATCPFthatthebindingenergyis367.47eV,whichisconsistentwithisinthevisible-lightregionat545nmwithmaximum.ThevaluesreportedforAgO.30Meanwhile,thesignalsofTi2pband-gapenergyofTCPFis3.1eVandtheband-gapenergyof23/2andTi2p1/2at458eVand464eVcorrespondingtoTiO2ATCPFpresentsamarkeddisplacementtolowerenergyvaluesThisjournalis©TheRoyalSocietyofChemistry2017RSCAdv.,2017,7,11211–11221|11215nViewArticleOnlineRSCAdvancesPaperFig.4Contactanglecharacterizationof(a)blankfilmand(b)ATCPF.at2.4eVapproximately,whichwereestimatedbytheKubelka–thatbothchitosanandTiO2areabundanthydrophilicgroup,Munkmethod(Fig.3f).32Thewidevisiblelightadsorptionandforexample,cOH.Inwatertreatment,thegoodhydrophilicityoflowbandgapinATCPFnanoclusterareallowetotheformationATCPFisconducivetowatermoleculediffusionandmassofp–nheterojunctionsasvisible-lightsensitization.Ag2Oisap-transfer,anditiseasytocombineanddegradepollutant.typenarrowbandgapsemiconductor,whileTiO2isann-typewidebandgapsemiconductor.AsshowninFig.3g,p–nheter-ojunctionsaresynthesisedattheinterfaceandelectrontransfer3.2PhotocatalyticactivitiesoccurredfromTiO2toAg2OwhentheirFermilevelsalign.AttheToprobeadvantagesofATCPF,adsorbingcapacityanddegra-equilibriumofjunction,thechargeofn-typeTiO2regionsisdationexperimentswerecarriedoutbyadsorbinganddecom-CreativeCommonsAttribution-NonCommercial3.0UnportedLicence.positive,whilethechargeofp-typeAg2Oisnegative.Therefore,posingtypicalcompounds:AMPandMO.Thesetwotypicalanopposingelectriceld(x,contactpotential)atthejunctioncompoundswerechosenasmodelorganicpollutantsbecauseandanequilibriumpotentialdifferenceV0areformedacrossantibioticandazodyesbothinvolveseriousrisksforthehumanthetransitionregion.Duringthephotocatalyticreactionbeingandaquaticenvironment.processofp-Ag2O/n-TiO2nanojunction,thephotogenerated3.2.1.Adsorptionanddegradationofampicillin(AMP).Aselectronsmovetotheconductionband(Ecn)ofthen-typeTiO2showninFig.5a,inthedark,ATCPFwasmoreefficientinandholesmovetothevalenceband(Evp)ofthep-typeAg2O.ThereducingtheconcentrationofAMPthanTCPF,CPFandAT,+chargeseparation(e/h)wereeffectivelybytrappingphoto-whichindicatedthatthesurfaceadsorptionpropertiesofThisarticleislicensedunderaelectronsonAg2OnanoparticlesandpowerfuloxidizingagentATCPFhadbeenobviousincreasedwhenTCPFwasmodiedby2andsuperoxideradical(OH,O,cOOH,cOH)wereproducedAg2O.ChitosanhadnoapparentadsorptioneffectonAMP,subsequentlyinaphotocatalysisdegradationreaction.whichwasprobablyduetothestericmacromolecular3.1.4.Watercontactanglemeasurement.InordertohindrancebetweenchitosanandAMP.AsshowninFig.5b,OpenAccessArticle.Publishedon13February2017.Downloadedon8/12/20181:13:00PM.understandthesurfacehydrophilicforphotocatalysisdegra-ATCPFexhibitedthehighestphotocatalyticactivityofMOdationandself-cleaningapplication,watercontactangleofdegradationundersimulatedsolarirradiation,thefollowingsampleswasanalyzed.AsshowninFig.4,thewatercontactseriesofphotoactivitywerefound:ATCPF>AT>TCPF>CPFangleofthebaresubstrateis68;however,thecontactangleofpolypropylenelm.ItwasclearthattheremovalofAMPbytheATCPFisonly38.Theresultdemonstratedthathydro-blankpolypropyleneber,CPFandATwerealldepressed,philicityofthelmtendstoincreaseaermodifying.Aplau-whichmeantthatAMPcannotbedegradeddirectlybyblanksiblemechanismforthisphenomenonhavebeensuggestedlmorchitosan.InthecaseoftheTCPFandAT,theFig.5(a)TheadsorptionofAMPindarkconditionwithinthepresenceofATCPF,TCPF,CPF,ATnanoparticlesandblankpolypropylenefilm;(b)1thedegradationofAMP(20mgL,40mL)duringsimulatedsolarirradiationinthepresenceofATCPF,TCPF,CPF,Ag2O/TiO2nanocomposites(AT)andblankpolypropylenefilm.11216|RSCAdv.,2017,7,11211–11221Thisjournalis©TheRoyalSocietyofChemistry2017nViewArticleOnlinePaperRSCAdvancesconcentrationofAMPcontinuedtodeclineastheirradiation3.2.3.ComparisonofreactionrateconstantofATCPFandtimewasprolonged;however,onlyabout40%ofAMPwasTCPF.Thereactionrateconstants(k)ofATCPFandTCPFweredegradedwithin3hinthepresenceofTCPorAT.Incompar-analyzedbyre-plottingthedegradationrateofAMPandMOison,ATCPFexhibitedaconsiderabledegradationabilityofaccordingtothefollowingequation:AMPundersimulatedsolarirradiation,andthedegradationdCrateofAMPreached100%in3h.ComparedwithCPF,the¼kCdtdistinguishedperformanceofATCPFshouldbeattributedtothegrastructureofAg2O/TiO2heterostructures,whichexposetocontactAMPinthesolutionandinducephotocatalyticAsshowninTable1,duringthedegradationprocessofAMPoxidationreaction.CombiningCPFwithAg2O/TiO2byself-andMOundersimulatedsolarirradiation,thevaluesofkassemblytoformATCPFalsopreventedtheinterferenceofindicatedthattherateconstantofATCPFwasmuchfasterthanchitosantoAMP,whichwasingoodagreementwiththesche-thatofTCP,CPFandAT.Thephotocatalysismechanismsofmaticstructure(Fig.2c).ThedegradationactivityofATCPFATCPFunderUV-andvisible-lightirradiationaredifferent.InundersimulatedsolarirradiationwasmuchhigherthanthetheUVrange,bothTiO2andAg2Oareexcitedtoproduceddarkcase,andthecorrespondingremovalratewas59.2%aerelectron–holepairs,andthenthegeneratedelectronsandholes3h.AlthoughATCPFplaysanimportantroleinreducingAMPcanreactwithH2Otoproducepowerfuloxidizingagentandsuperoxideradical.35Inthevisiblerange,onlyAgOcanbewithoutirradiation,thelighttreatmentwasconsideredneces-2sarytoimprovethephotocatalyticperformanceofATCPF.excitedtoproduceelectron–holepairs,whichthenactas3.2.2.DegradationofMO.Tofurtherevaluatethemulti-visible-lightactivecomponenttoenhancetheAg2O/TiO2pho-functionalityofATCPF,theadsorptionanddecompositionoftocatalyticactivitytodecomposeorganicpollutants.Therefore,MOwereexamined,asshowninFig.6.Inthedarkcondition,thesetwodifferentmechanismsunderUV-visiblelightjointlyATCPFstillperformedgooddecolorizationofMO.ItwasfoundpromotedthephotocatalyticprocessofATCPF.CreativeCommonsAttribution-NonCommercial3.0UnportedLicence.thatblankpolypropylenelmandATagainhadnoeffecton3.2.4.Mineralizationstudies.ThemineralizationeffectsMO,whereasCPFpossessedabsorptivityandreachedsatura-wereanalyzedbystudyingtheTOCcontentsofreactionsolutiontionabout34.6%in15min.Thisphenomenonwasslightlyduringphotocatalyticreaction.AerreactionwithATCPFfordifferentfromthecaseofAMP,whichwasduetotheinteraction15min,thedegradationrateofMOwas96.00%andtheremovalbetweenthefunctionalgroups(hydroxylandamine)ofchitosanrateofTOCwas83.77%(Fig.7a).Itdemonstratedthatminer-andMO.33AerthesurfaceofCPFwascoatedwithTiO,thealizationisasynchronouslyprocesswithdegradationreaction.2obtainedTCPFexhibitedaslightlystrongeradsorptioncapacityTheresultsfurtherindicatedthattheas-preparedATCPFcanbeofMOinthedark,andabout50.3%decolorizationwasusedasahighlyefficientmineralizationphotocatalystthatcanattained,indicatingthatTiO2alsohadaffinityforMOowingtomineralizedyeefficientlywithsimulatedsunlight.However,asThisarticleislicensedunderathehydroxylgroupsonthesurfaceofTiO2.34WhenexposedtoshowninFig.7b,aerreactionwithTCPFfor15min,thevisiblelight,photocatalyticactivityofTCPwasmoreefficientdegradationrateofMOwas73.41%,whiletheTOCremovalratethanthatofCP.Itcouldbeattributedtoboththeadsorptionofwasonly24.75%.Theresultsdemonstratedthattheminerali-CPFandphotocatalysisofATundersimulatedsolarirradiation.zationprocesswassignicantlyimprovedbecauseoftheOpenAccessArticle.Publishedon13February2017.Downloadedon8/12/20181:13:00PM.SimilartothecaseofATCPFfordegradingAMP,thephoto-excellentphotocatalysisofAg2O.catalysisdegradationofMObyATCPFoccurredatasigni-3.2.5.Photocatalyticantibacterialcharacteristics.Toeval-cantlyhigherratethanthatbyTCPF,thoroughlycompleteduatetheantibacterialactivityoftheAg2O/TiO2modiedwithin30minundervisiblelightirradiation.Theaboveresultschitosan-basedphotocatalyticlm,thebactericidalefficiencyoffurtherindicatedthattheas-preparedATCPFcanbeusedasATCPFwascomparedtotheactivitiesoftheotherphoto-ahighlyefficientphotocatalystthatcanusesimulatedsunlight.catalystsmaterials.AsshowninFig.8a,anegligible1Fig.6(a)TheadsorptionofMO(10mgL,40mL)indarkconditionwithinthepresenceofATCPF,TCPF,CPF,ATandblankfilmpolypropylene.1(b)ThedegradationofMO(10mgL,40mL)duringsimulatedsolarirradiationinthepresenceofATCPF,TCPF,CPF,ATandblankpoly-propylenefilm.Thisjournalis©TheRoyalSocietyofChemistry2017RSCAdv.,2017,7,11211–11221|11217nViewArticleOnlineRSCAdvancesPaperTable1TherateconstantduringphotocatalyticdegradationofAMPandMOPollutantssystems(under41414141simulatedsolarirradiation)kATCPF(10min)kTCPF(10min)kCPF(10min)kAT(10min)1AMP(C0¼20mgL)196270.11381MO(C0¼10mgL)1643214891Fig.7ConcentrationchangeandTOCanalysisof(a)ATCPF,(b)TCPFduringphotocatalyticdegradationofMO(C0MO¼10mgL;V¼40mL;M¼0.075g;N¼70rpm;UV;t¼6h).CreativeCommonsAttribution-NonCommercial3.0UnportedLicence.antibacterialactivitywasobservedintheabsenceofphoto-undervisiblelight(Fig.8c),itwasobservedthatPF,CPFandcatalyst(curve1).BecauseofthelowerphotocatalyticactivityofTCPFdidnotshowanyantimicrobialactivitiesbyvisiblelight;ATandCPFundervisiblelight,only56%and35%inactivationhowever,ATCPFshowedapalpableDIZvaluewhichindicatingofE.coliwasfoundevenaer60minofirradiation(curve3andsignicantantibacterialactivityagainstE.coliwithaconcen-414).Incontrast,obviousenhancementsinvisible-lightbacteri-trationsofapproximately10CFUmL.ItcanbepredictedcidalefficiencywasobservedonTCPF(curve5)andATCPFthattheexcellentbiocidalfunctionofATCPFisderivedfromthe(curve6).Inparticular,99.8%oftheE.coliwaskilledontheAgspeciesandtheoxidativestresscausedbyphoto-generatedATCPFuponirradiationfor60min(curve6).Additionally,byreactiveradicals.6,29ThisarticleislicensedunderacomparingthebactericidalresultsonATCPFinthepresence3.2.6.Multiplereuseability.Toinvestigatethestabilityof(curve6)andabsence(curve2)ofvisible-lightirradiation,itisATCPFonthephotocatalyticactivityundersimulatedsolar+obviousthat,apartfromtheeffectoftheAgions,themainlyirradiation,thesamesampleswerereusedforvetimestobactericidaleffectsisrelatedtothephotocatalyticprocess.AsremoveMO,andresultswereshowninFig.9.Forcomparison,OpenAccessArticle.Publishedon13February2017.Downloadedon8/12/20181:13:00PM.showninFig.8b,theantibacterialactivityofATCPFwasalsotherepeateddegradationexperimentswerealsocarriedoutinassessedbythediameterofinhibitionzone(DIZ)testwiththedarkcondition.Underirradiation,itwasfoundthatMOcanvariousconcentrationsofE.coil.FromtheresultsofDIZtestbefullydegradedwithin30mininthersttwocycles.AlongFig.8(a)KineticsofbactericidalefficiencyagainstE.coliundervisible-lightirradiation:(1)E.colisuspensionwithoutanycatalystirradiatedbyvisiblelight,(2)E.colisuspensionswith0.1gofATCPFinthedark,(3–6)E.colisuspensionswith0.1gof(3)CPF,(4)AT,(5)CTPFand(6)ACTPF.(b)Comparativestudyofthezoneofinhibition(mm)forATCPFinE.colibacterialstrainundervisible-lightirradiation.(c)Photographsofanti-bacterialresultsonE.coliforATCPF.11218|RSCAdv.,2017,7,11211–11221Thisjournalis©TheRoyalSocietyofChemistry2017nViewArticleOnlinePaperRSCAdvancesFig.9(a)Repeatedadsorption–photocatalyticdegradationexperimentscarriedoutundersimulatedsolarirradiationandindarkshowingthe1photodegradationefficiencyandrecyclabilityofATCPFcompositesinthedegradationofMO(C0¼10mgL,V¼40mL).(bandc)Ionic4++concentrationandreleaseamountofTiandAgduringreusedforfivetimes.CreativeCommonsAttribution-NonCommercial3.0UnportedLicence.withtheincreaseofreusingtimes,thephotocatalyticefficiencyThepossiblereleaseamountofAgionandTiionintheofATCPFdecreasesslightly.ThismightbebecauseofthesolutionaerATCPFwasreuseddifferenttimesweredeter-presumedlossofAg2O/TiO2nanoparticlesorbecauseofsomeminedbyICP-MS,andtheresultsareshowninFig.9bandc.4+degradationintermediatesthatcoverthesurfaceoftheas-Comparedwithcontrolgroup,anegligibleconcentrationofTipreparedsamples.Nevertheless,thedegradationrateofMOwasdetectedduringreusedforvetimes,whichdemonstratedstillremainsover90%aerreusing5times.However,inthethatTiO2nanoparticlesweremodiedonthesurfaceofCPF1+caseofdarkapplication,thedegradationefficiencyofATCPFrmly.Whileabout1mgLAgwasdetectedduringformerseverelydiminisheswiththeincreaseofrepeateduse.Oncethreetimesreusing.ItcanbepredictedthattheincreaseofThisarticleislicensedundera+reachingadsorptionequilibrium,theconcentrationofMOreleasedamountofAgledtothedecreaseofphotocatalyticwouldnolongerdecrease.Therefore,irradiationseemstobeactivity.necessarytokeepthehighphotocatalyticactivityandstabilityofTheobservedadsorptionperformance,photocatalyticOpenAccessArticle.Publishedon13February2017.Downloadedon8/12/20181:13:00PM.ATCPF.Inaddition,ATCPcanbeeasilyseparatedandreusedperformanceandstablereusabilityarefarbetterthanmostofdirectlywithoutextraregenerationprocesses.thereportednanostructureswhichhasbeensummarizedinTable2PerformanceofphotocatalyticactivityinliteratureCatalystusedConc.andLightsourceTimeDegradationAdsorptionRepeatedandamountvolumeofMO(W)(min)(%)(%)photooxidation(%)Reference1thAg2O/TiO2photocatalytic10mgL,40mL150(vis)15966490(5)Ourworklm(10mg)1Ag2O/TiO2nanobelt20mgL,10mL350(vis)80863—36(10mg)1thAg2O/TiO2nanobelts20mgL,20mL300(vis)2580—76(4)37(20mg)1thS-DopedAg2O/TiO2nanobelt20mgL,20mL300(vis)15080—80(6)38(20mg)1Ag2O/TiO2microsphere14mgL,50mL500(vis)6099——39(20mg)1thAg2O/TiO2nanosheet160mgL,100mL160(UV)602087.578(5)40(100mg)1thAg2Onanocomposites16mgL,10mL500(UV)8985100(8)41(30mg)1thAg2O/TiO2microspheres3mgL,40mL40(UV)1593393(5)42(40mg)Thisjournalis©TheRoyalSocietyofChemistry2017RSCAdv.,2017,7,11211–11221|11219nViewArticleOnlineRSCAdvancesPaperTable2.Therefore,thepreparedATCPcanberegardedasinthepresenceofgoldnanoparticlesloadedTiO2underahighlyactive,easilyseparatedandhighlystablesunlightUV-visiblelight,Chem.Eng.J.,2014,241,401–409.photocatalyst,whichshowsagreatpotentialintheeldof6G.Xiao,X.Zhang,W.Zhang,S.Zhang,H.SuandT.Tan,environmentalremediationthroughitssimplicityandlow-cost.Visible-light-mediatedsynergisticphotocatalyticantimicrobialeffectsandmechanismofAg-4.Conclusionsnanoparticles@chitosan–TiO2organic–inorganiccompositesforwaterdisinfection,Appl.Catal.,B,2015,BasedonthesynergisticcatalytictechniqueofAg2OandTiO2170,255–262.semiconductors,thesunlightactiveAg2O/TiO2-modied7M.M.MohamedandM.S.Al-Sharif,Visiblelightassistedchitosan-basedphotocatalyticlmwassuccessfullyprepared.reductionof4-nitrophenolto4-aminophenolonAg/TiO2FESEM,HRTEM,XRDandXPSwereusedforthecharacteriza-photocatalystssynthesizedbyhybridtemplates,Appl.tionofATCPF,illustratingthatAg2OnanoparticleswererstlyCatal.,B,2013,142,432–441.depositedonTiO2nano-clusterandthenAg2O/TiO2wasas8L.Xiang,X.Zhao,C.ShangandJ.Yin,AuorAgnanoparticle-awholeimmobilizedonchitosan-modiedpolypropyleneber,decorated3Durchin-likeTiO2nanostructures:synthesis,amultilayercompositewithgra-likestructurewasobtained.characterization,andenhancedphotocatalyticactivity,J.ThehydrophilicATCPFlmdisplayedexcellentabsorptionColloidInterfaceSci.,2013,403,22–28.capacitybymodiedchitosan,TiO2andAg2O.Becauseofits9A.V.RosarioandE.C.Pereira,TheroleofPtadditiononthevisible-lightabsorptionandlowbandenergy,theATCPFlmphotocatalyticactivityofTiO2nanoparticles:thelimitshowedexcellentphotocatalyticpropertytowardsthecompletebetweendopingandmetallization,Appl.Catal.,B,2014,degradationoftwodifferenttargetorganics:AMPandMO,144,840–845.within180minand30min,respectively.ATCPFcanbereused10R.Molinari,C.LavoratoandP.Argurio,Photocatalyticdirectlyfor5timeswithitsphotocatalyticefficiencyforMOreductionofacetophenoneinmembranereactorsunderCreativeCommonsAttribution-NonCommercial3.0UnportedLicence.remaininginexcessof90%,whichensuredagoodstabilityandUVandvisiblelightusingTiO2andPd/TiO2catalysts,recyclabilityofthecomposite.ThepresentstudydevelopedanChem.Eng.J.,2015,274,307–316.efficientsunlightactivatedphotocatalyst,anditisofrealistic11M.Kim,Y.K.Kim,S.K.Lim,S.KimandS.I.In,Efficientsignicanceinwastewatertreatmentaswellasscienticvisiblelight-inducedH2productionbyAu@CdS/TiO2signicanceindesigningvisiblelightactivenano-nanobers:Synergisticeffectofcore–shellstructuredphotocatalysts.Au@CdSanddenselypackedTiO2nanoparticles,Appl.Catal.,B,2015,166,423–431.Acknowledgements12W.Zhou,H.Liu,J.Wang,D.Liu,G.Du,S.Han,J.LinandR.Wang,InterfacedominatedhighphotocatalyticThisarticleislicensedunderaTheauthorsexpresstheirthanksforthesupportsfromthepropertiesofelectrostaticself-assembledAg2O/TiO2NationalNaturalScienceFoundationofChina(21525625),theheterostructure,Phys.Chem.Chem.Phys.,2010,12,15119–NationalBasicResearchProgram(973Program)ofChina15123.OpenAccessArticle.Publishedon13February2017.Downloadedon8/12/20181:13:00PM.(2014CB745100),the(863)HighTechnologyProject13J.LichtenbergerandM.D.Amiridis,Catalyticoxidationof(2013AA020302)andtheChineseUniversitiesScienticFundchlorinatedbenzenesoverV2O5/TiO2catalysts,J.Catal.,(JD1417).2004,223,296–308.14T.Arai,M.Horiguchi,M.Yanagida,T.Gunji,H.SugiharaReferencesandK.Sayama,ReactionmechanismandactivityofWO3-catalyzedphotodegradationoforganicsubstances1R.ChalasaniandS.Vasudevan,Cyclodextrin-functionalizedpromotedbyaCuOcocatalyst,J.Phys.Chem.C,2009,113,Fe3O4@TiO2:reusable,magneticnanoparticlesfor6602–6609.photocatalyticdegradationofendocrine-disrupting15Y.Huo,X.Chen,J.Zhang,G.Pan,J.JiaandH.Li,Orderedchemicalsinwatersupplies,ACSNano,2013,7,4093–4104.macroporousBi2O3/TiO2lmcoatedonarotatingdisk2V.Vaiano,O.Sacco,D.SanninoandP.Ciambelli,withenhancedphotocatalyticactivityundervisibleNanostructuredN-dopedTiO2coatedonglassspheresforirradiation,Appl.Catal.,B,2014,148,550–556.thephotocatalyticremovaloforganicdyesunderUVor16X.Wang,S.Li,H.Yu,H.Yu,J.YuandS.Liu,Ag2OasaNewvisiblelightirradiation,Appl.Catal.,B,2015,170,153–161.Visible-LightPhotocatalyst:Self-StabilityandHigh3J.Zhang,W.Wu,S.Yan,G.Chu,S.Zhao,X.WangandC.Li,PhotocatalyticActivity,Chem.–Eur.J.,2011,17,7777–7780.Enhancedphotocatalyticactivityforthedegradationof17G.Wang,X.Ma,B.Huang,H.Cheng,Z.Wang,J.Zhan,rhodamineBbyTiO2modiedwithGd2O3calcinedathighX.Qin,X.ZhangandY.Dai,ControlledsynthesisofAg2Otemperature,Appl.Surf.Sci.,2015,344,249–256.microcrystalswithfacet-dependentphotocatalytic4E.Schuler,A.KGustavsson,S.HertenbergerandK.Sattler,¨activities,J.Mater.Chem.,2012,22,21189–21194.SolarphotocatalyticandelectrokineticstudiesofTiO2/Ag18M.Xu,L.HanandS.Dong,Facilefabricationofhighlynanoparticlesuspensions,Sol.Energy,2013,96,220–226.efficientg-C3N4/Ag2Oheterostructuredphotocatalystswith5N.Pugazhenthiran,S.Murugesan,P.Sathishkumarandenhancedvisible-lightphotocatalyticactivity,ACSAppl.S.Anandan,PhotocatalyticdegradationofceiofursodiumMater.Interfaces,2013,5,12533–12540.11220|RSCAdv.,2017,7,11211–11221Thisjournalis©TheRoyalSocietyofChemistry2017nViewArticleOnlinePaperRSCAdvances19R.Liu,P.Wang,X.Wang,H.YuandJ.Yu,UV-andvisible-31G.Xiao,H.SuandT.Tan,Synthesisofcore–shellbioaffinitylightphotocatalyticactivityofsimultaneouslydepositedchitosan–TiO2compositeanditsenvironmentalanddopedAg/Ag(I)–TiO2photocatalyst,J.Phys.Chem.C,applications,J.Hazard.Mater.,2015,283,888–896.2012,116,17721–17728.32S.Oros-Ruiz,R.ZanellaandB.Prado,Photocatalytic20J.Zou,Y.Xu,B.Hou,D.WuandY.Sun,Self-assemblyAg2Odegradationoftrimethoprimbymetallicnanoparticlesnanoparticlesintonanowireswiththeaidofamino-supportedonTiO2-P25,J.Hazard.Mater.,2013,263,28–35.functionalizedsilicananoparticles,PowderTechnol.,2008,33B.Tanhaei,A.Ayati,M.LahtinenandM.Sillanp¨a¨a,183,122–126.Preparationandcharacterizationofanovelchitosan/Al2O3/21D.Li,H.Zheng,Q.Wang,X.Wang,W.Jiang,Z.Zhangandmagnetitenanoparticlescompositeadsorbentforkinetic,Y.Yang,Anoveldouble-cylindrical-shellphotoreactorthermodynamicandisothermstudiesofmethylorangeimmobilizedwithmonolayerTiO2-coatedsilicagelbeadsadsorption,Chem.Eng.J.,2015,259,1–10.forphotocatalyticdegradationofrhodamineBandmethyl34Z.Zainal,L.K.Hui,M.Z.HusseinandA.H.Abdullah,orangeinaqueoussolution,Sep.Purif.Technol.,2014,123,CharacterizationofTiO2–chitosan/glassphotocatalystfor130–138.theremovalofamonoazodyeviaphotodegradation–22F.Shiraishi,A.MiyawakiandR.Chand,Amechanismoftheadsorptionprocess,J.Hazard.Mater.,2009,164,138–145.photocatalyticdecompositionof2,4-dinitrophenolonTiO235W.J.Zhou,Y.H.Leng,D.M.Hou,H.D.Li,L.G.Li,G.Q.Li,immobilizedonaglasssurface,Chem.Eng.J.,2015,262,H.LiuandS.W.Chen,Phasetransformationandenhanced831–838.photocatalyticactivityofS-dopedAg2O/TiO223T.T.Vu,T.Vald´es-Sol´ısandG.Marb´an,Highsurfaceareaheterostructurednanobelts,Nanoscale,2014,6,4698–4704.stainlesssteelwiremesh-supportedTiO2preparedby36N.Wei,H.Cui,Q.Song,L.Zhang,X.Song,K.Wang,sacricialtemplateacceleratedhydrolysis.AmonolithicY.Zhang,J.Li,J.WenandJ.Tian,Ag2Onanoparticle/TiO2photocatalystsuperiortoP25TiO2,J.Environ.Chem.Eng.,nanobeltheterostructureswithremarkablephoto-responseCreativeCommonsAttribution-NonCommercial3.0UnportedLicence.2014,2,2229–2235.andphotocatalyticpropertiesunderUV,visibleandnear-24Z.Wang,K.Yoshinaga,X.R.BuandM.Zhang,Lowinfraredirradiation,Appl.Catal.,B,2016,198,83–90.temperaturefabrication&photocatalyticalactivityof37W.Zhou,H.Liu,J.Wang,D.Liu,G.DuandJ.Cui,Ag2O/TiO2carbonber-supportedTiO2withdifferentphasenanobeltsheterostructurewithenhancedultravioletandcompositions,J.Hazard.Mater.,2015,290,134–141.visiblephotocatalyticactivity,ACSAppl.Mater.Interfaces,25D.Li,Q.Zhu,C.Han,Y.Yang,W.JiangandZ.Zhang,2010,2,2385–2392.Photocatalyticdegradationofrecalcitrantorganic38W.Zhou,Y.Leng,D.Hou,H.Li,L.Li,G.Li,H.Liuandpollutantsinwaterusinganovelcylindricalmulti-columnS.Chen,PhasetransformationandenhancedphotoreactorpackedwithTiO2-coatedsilicagelbeads,J.photocatalyticactivityofS-dopedAg2O/TiO2ThisarticleislicensedunderaHazard.Mater.,2015,285,398–408.heterostructurednanobelts,Nanoscale,2014,6,4698–4704.26S.Singh,H.MahalingamandP.K.Singh,Polymer-39F.Chen,Z.Liu,Y.Liu,P.FangandY.Dai,Enhancedsupportedtitaniumdioxidephotocatalystsforadsorptionandphotocatalyticdegradationofhigh-OpenAccessArticle.Publishedon13February2017.Downloadedon8/12/20181:13:00PM.environmentalremediation:areview,Appl.Catal.,A,2013,concentrationmethyleneblueonAg2O-modiedTiO2-462,178–195.basednanosheet,Chem.Eng.J.,2013,221,283–291.27C.D.Tran,S.Duri,A.DelneriandM.Franko,Chitosan-40H.Hua,Y.Xi,Z.Zhao,X.Xie,C.HuandH.Liu,Gram-scalecellulosecompositematerials:preparation,wetchemicalsynthesisofAg2O/TiO2aggregatedspherecharacterizationandapplicationforremovalofheterostructurewithhighphotocatalyticactivity,Mater.microcystin,J.Hazard.Mater.,2013,252,355–366.Lett.,2013,91,81–83.28D.Delai´aSun,Facilefabricationofporouschitosan/TiO2/41W.Jiang,X.Wang,Z.Wu,X.Yue,S.Yuan,H.LuandFe3O4microsphereswithmultifunctionforwaterB.Liang,SilverOxideasSuperbandStablePhotocatalystpurications,NewJ.Chem.,2011,35,137–140.underVisibleandNear-InfraredLightIrradiationandIts29P.Magesan,S.SanujaandM.J.Umapathy,NovelhybridPhotocatalyticMechanism,Ind.Eng.Chem.Res.,2015,54,chitosanblendedMoO3–TiO2nanocompositelm:832–841.evaluationofitssolarlightphotocatalyticandantibacterial42D.Sarkar,C.K.Ghosh,S.Mukherjeeandactivities,RSCAdv.,2015,53,42506–42515.K.K.Chattopadhyay,ThreedimensionalAg2O/TiO2type-II30C.D.Wagner,HandbookofX-rayphotoelectronspectroscopy:(p–n)nanoheterojunctionsforsuperiorphotocatalyticareferencebookofstandarddataforuseinX-rayactivity,ACSAppl.Mater.Interfaces,2012,5,331–337.photoelectronspectroscopy,PhysicalElectronicsDivision,Perkin-ElmerCorp,1979.Thisjournalis©TheRoyalSocietyofChemistry2017RSCAdv.,2017,7,11211–11221|11221



